HEMOGLOBIN STRUCTURE
1. What are hemoglobins?
These are an integral component of blood that plays a major role in O2 tranport and helps in providing viscosity, deformability to RBCs.
2. What is structure of Hemoglobin?
It consists of a pair of α like polypeptide globin chain and a pair of β like polypeptide chain.
3. Where are genes for α and β polypeptide located ?
α gene are located in chromosome 16 and β in chromosome 11.
There are 4 α genes and 2 β genes.
 |
4. What is heme?
HEME is
Iron is in the reduced or ferrous form (Fe2+) and it is only in this form iron can carry oxygen
Each globin chain contain 1 heme moiety.
Each heme moiety contains 1 Fe2+
Each Fe2+ can carry 1 oxygen molecule
So 1 hemoglobin molecule can carry 4 oxygen molecule. |
5. What are the different types of hemoglobin?
Over different age groups from embryonic to fetus to children and to adults 6 different types of hemoglobin are identified
Embryonic hemoglobins (Gower-1,Gower-2, and Portland),
Fetal hemoglobin (HbF), and
Adult hemoglobins (HbA and HbA2 ).
The electrophoretic mobilities of hemoglobins vary with their chemical structures.
 |
| Source; Nelson Textbook of Pediatrics |
6. In embryos up to 6 wk gestation, the Gower hemoglobins predominate but are no longer detectable by 3 mo of gestation.
7. By 6-8 wk gestation, HbF (α2 γ2 ) is the predominant hemoglobin;
At 24 wk gestation it constitutes 90% of the total hemoglobin.
HbF declines modestly in the third trimester, such that the HbF comprises 70–80% of the total hemoglobin.
HbF production decreases rapidly postnatally and by 6-12 mo of age reaches adult levels of <2%.
8. What is responsible for the switch from fetal to adult hemoglobin?
The transcription factor BCL11A, which binds to the β-globin gene and acts to silence γ-globin expression and thus HbF.
9. What are the different structures of hemoglobin?
· Primary structure consists of linear sequence of polypeptides.
· Secondary structure is highly helical structure.
· Tertiary structures cause the exterior surfaces to be rich in polar (hydrophilic) amino acids that enhance solubility, and the interior to be lined with nonpolar groups, forming a hydrophobic pocket into which heme is inserted.
· Quaternary Structures
10.What are quaternary structures?
· Tetrameric quaternary structure of HbA contains two αβ dimers. Numerous tight interactions (i.e., α1β1 contacts) hold the α and β chains together.
· The complete tetramer is held together by interfaces (i.e., α1β2 contacts) between the α-like chain of one dimer and the non-α chain of the other dimer.
11. What makes the hemoglobin soluble ?
Tetramer structure and the αβ contacts make it soluble.
However individual globin chain aren’t soluble and in the absence of corresponding globin chain precipitates damaging the RBCs.
E.g. in Thalassemia when beta chain is not formed the extra alpha chains gets precipitated damaging RBCs and causing ineffective hemolysis.
12. What are the types of adult hemoglobin?
2 types
· HbA
· HbA2
Adult hemoglobin HbA reaches to 30% by term and by 6-12 months of age HbA reaches adult level
At birth HbA2 constitutes <1% of total HB
By 12 months it reaches to adult level of 2.0–3.4%.
Throughout life, the normal ratio of HbA to HbA2 is about 30 : 1.
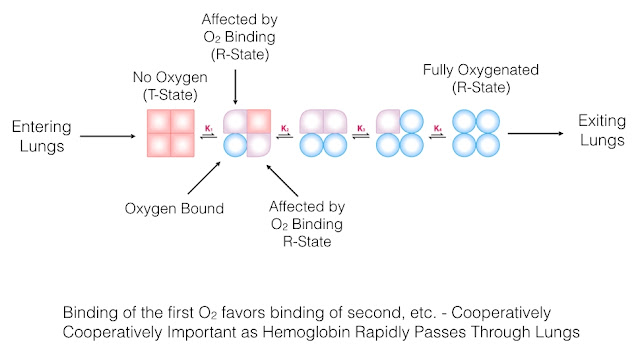
13.What is cooperative O2 binding of Hemoglobin?
It is the phenomenon in which binding of O2 to one site in Hb increases the O2 affinity of the remaining sites.
Similarly release of O2 from 1 site increases the probability of release of o2 from other sites
This helps in binding of O2 at the lungs and release at the tissue.
14. What are R and T forms of Hb?
T is tensed form of Hb and is a form which has low oxygen affinity that is when no O2 is bound. (low cooperative state).
R is relaxed form which indicates Hb with high affinity for O2.
When Hb binds first O2 molecule it has low affinity but after binding that O2 molecule affinity increases a phenomenon called cooperative O2 binding.
So when 3 molecules are bound to Hb the T form is transformed to R form which has the highest affinity.


Comments
Post a Comment