Congenital Hypothyroidism: Screening and Management (AAP 2022 Recommendations)
Hypothyroidism
Newborn screening (NBS) for CH followed by prompt initiation of levothyroxine (L-T4) therapy can prevent severe intellectual disability, psychomotor dysfunction, and impaired growth.
The influence of severity at diagnosis, timing of treatment initiation, and L-T4 dose on longterm outcomes remains a matter of debate.
Although the majority of treated patients achieve normal cognitive outcomes, the influence of L-T4 therapy on neurologic development is less certain, and some studies show persistent deficits in treated patients compared with euthyroid healthy controls.
If a permanent form of CH has not been established, L-T4 treatment is maintained until 3 years of age, after which thyroid function is reevaluated following a 4- to 6-week discontinuation of L-T4.
Most common cause - thyroid dysgenesis, dyshormonogenesis.
Less common cause - maternal antithyroid medications, TSH receptor-blocking antibodies (TRBAb) acquired through transplacental passagematernal or infant iodine deficiency or excess.
Rarely, CH is caused by hypothalamic or pituitary defects that lead to inadequate secretion and/or bioactivity of TSH (central hypothyroidism).
The fetal thyroid begins to form around 3 weeks of gestation and begins to synthesize TH at around 10 to 12 weeks of gestation.
Hypothalamic and pituitary control of the thyroid is asserted around midgestation and develops through the third trimester, reaching maturity around term gestation.
Intake of a prenatal vitamin containing 150 mcg of iodine daily by all women before and during pregnancy and lactation will promote iodine sufficiency in mother and newborn infant
The incidence of CH ranges from approximately 1 in 2000 to 1 in 4000 newborn infants in countries from which NBS data are available.
A dried blood spot (DBS) for NBS is obtained by heel stick on an approved filter paper card using appropriate collection methods. After drying, filter paper specimens.
PREFERRED AGE FOR SAMPLE COLLECTION:
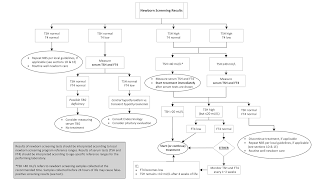
The preferred age for collection of the NBS specimen to screen for CH is 48 to 72 hours of age. This timing reflects the normal surge in TSH concentration (to 60–80 mIU/L) that occurs within hours after birth in term newborn infants and resolves over the next 5 days.
Specimens collected in the first 24 to 48 hours of life may lead to false-positive TSH - so avoid taking samples before 48 hrs.
Conditions when sample can be taken before 48 hrs:
- 90% of newborns are discharged before 48 hours so shall be taken rather than to miss.
- Before blood transfusion.
NBS test priority
- Chief priority - to identify moderate to severe primary hypothyroidism
- Secondary priority - mild primary hypothyroidism, primary hypothyroidism of delayed onset ( delayed TSH elevation), central hypothyroidism.
NBS programs may measure TSH, T4 from whole blood or serum samples.
Based on the assumption that the average newborn hematocrit is 55%, results expressed in serum units are approximately 2.2-fold those expressed in whole blood units; for example, a screening TSH of 15 mIU/L in whole blood is roughly equivalent to a TSH of33 mIU/L serum.
One must keep in mind about the serum and whole blood samples.
Test strategies
(a) primary TSH – reflex T4 measurement;
(b) primary T4 – reflex TSH measurement; and
(c) combined T4 and TSH measurement
Primary TSH – reflex T4 measurement
First TSH is measured - if elevated total T4 is measured from the sample.
Depending on the NBS program algorithm, an elevated TSH and/or low T4 may lead to recall for a confirmatory serum sample or to collection of a second DBS specimen for repeat testing.
Adv -
Can detect mild cases
Disadvantage -
Cannot detect hypothyroidism with delayed onset TSH elevation and central hypothyroidism.
Primary T4- reflex TSH strategy
Total T4 measured first - if low do TSH from same sample
if T4 low and high TSH do serum confirmatory or second DBS specimen is collected.
Adv -
can detect delayed TSH elevation because the infants at highest risk for delayed TSH elevation are also likely to have low total T4 concentrations on initial screen
can detect central hypothyroidism as T4 will be low with central causes
Disadvantage -
cannot detect mild cases of congenital hypothyroidism.
Combined testing strategy -
Combines adv of both and supplants disadvantages
NBS in special populations-
Preterm or LBW — low serum T4 and reduced TSH surge
- Multifactorial causes of hypothyroidism in preterm/ LBW - early loss of maternal T4, decreased TBG, low thyroid reserve, immature HPT axis, inadequate iodine in nutrition, nonthyroidal illness (low T4 with normal to low TSH).
- low T4 with normal TSH is called hypothyroxinemia which is detected by primary T4 strategy — this is transient.
- Although TSH concentrations are often normal or low in preterm or LBW infants, as they recover from hypothyroxinemia serum, TSH levels may rise transiently above normal (6–15 mIU/L).
- In some infants, this increase in TSH is significantly greater (>100 mIU/L). This pattern of “delayed TSH rise” is more common in preterm or LBW infants than in term or normal birth weight infants.
- Many NBS programs have elected to undertake repeat NBS in infants born preterm (<32weeks’ gestation) or with VLBW (<1500 g).
- For routine rescreening of these infants, repeat NBS testing is preferred to measurement of serum TSH and FT4 because of the much lower cost of NBS.
Acute Illness or Admission to a NICU
- Risk of non thyroidal illness
- risk of drugs that can affect thyroid function → low TSH
- Also risk of transient hypothyroximenia with low T4 and normal TSH
- Risk of delayed surge of TSH
Multiple births/same sex twins / in vitro fertilization
- High incidence of CH in multiple births
- Most twin pairs (>95%) are discordant for CH.
- However, in monozygotic twins who share placental circulation, blood from a euthyroid fetal twin with normal TH levels may cross to a fetal twin with CH, temporarily correcting the hypothyroidism and preventing its detection by initial NBS at 24 to 72 hrs.
- All monozygotic or same sex twins for whom zygosity is unknown should repeat NBS around 2 weeks.
- Also delayed elevation of TSH at 2 to 4 weeks has been seen in cases of in vitro fertilization - however insufficient evidence available to recommend repeat sample.
Trisomy 21
- At significant risk of hypothyroidism - have low T4 and high TSH d/t unknown reasons - also have risk of other illness that can contribute to non thyroidal illness - excess iodine exposure in heart disease which is common in trisomy 21.
- 2nd NBS should be performed at 2 to 4 weeks of life and serum TSH should be measured at 6 and 12 months of life.
- Hypothyroidism may develop before 6 months in some infants with trisomy so a high level of clinical suspicion remains important, but there is insufficient evidence to suggest additional routine TSH screening.
What next after abnormal NBS?
Physical exam - goiter/lingual thyroid and other stigmata of CH
serum TSH, FT4
fT3, rT3, total T3 is rarely required.
If TBG binding abnormality is suspected - do T4
Interpretation
- If the confirmatory serum TSH is >20 mIU/L, L-T4 treatment should be initiated even if FT4 is normal.
- If the confirmatory serum TSH is elevated but #20 mIU/L, treatment may be initiated, or alternatively serum TSH and FT4 may be followed closely every 1 to 2 weeks without treatment.
- However, repeated TSH elevation above 10 mIU/L has been associated with adverse outcomes in children with CH. Therefore, expert opinion suggests that persistent TSH elevation >10 mIU/L is an indication to initiate L-T4 treatment.
Gray area:
- The management of infants with persistent serum TSH elevations between 5 and 10 mIU/L after 4 weeks of age is even more controversial. The upper limit of normal TSH in infants age 1 to 3 months is around 4.1 to 4.8 mIU/L.
- TSH elevation above 5 mIU/L beyond 1 to 3 months of life is generally abnormal but does not demonstrate that L-T4 treatment is necessary or beneficial. Thus, in infants with serum TSH elevation between 5 and 10 mIU/L that persists beyond 4 weeks of age, there is insufficient evidence to support treatment versus observation.
IMAGING
- Controversy exists regarding the utility of routine thyroid imaging for patients with CH.
- It may inform prognosis if it identifies an ectopic or a dysgenic thyroid gland suggesting a permanent form of CH, or if it guides counseling on the likelihood of disease recurrence in a future child of the same parents.
- However, in most cases imaging does not alter clinical management of the patient before age 3 years.
- USG of thyroid has low sensitivity than scintigraphy but can be improved with addition of doppler but has no radiation exposure.
- Thyroid scintigraphy allows localization of functional thyroid tissue based on its uptake of either 123I or 99mTc.
- 123I may provide more accurate uptake and images but may not be available in all imaging centers. 99mTc is less expensive and is widely available.
- 131I is not used in infants because of the higher exposure to ionizing radiation.
- Iodine uptake in an enlarged, eutopic gland is consistent with a defect in TH synthesis. Measurement of serum thyroglobulin will distinguish a thyroglobulin synthesis defect from other genetic causes of dyshormonogenesis or exposure to an exogenous goitrogen other than iodine, such as antithyroid drugs.
Genetic testing:
Genetic information may be perceived as valuable by families, but genetic testing can present challenges of cost and interpretation of indeterminate results, and in many cases a genetic diagnosis may not alter clinical.
Treatment:
- L-T4 at a starting dose of 10 to 15 mcg/kg per day, administered once daily.
- L-T4 tablets are the treatment of choice.
- Commercial oral solution of L-T4 is approved by the US Food and Drug Administration for use in children of any age; however, limited experience with its use showed that dosing may not be equivalent to dosing with tablet formulations.
- L-T4 suspensions prepared by compounding pharmacies may result in unreliable dosing.
- Breastfeeding doesn't alter the drug intake.
- If enteral administration is not possible, L-T4 can be administered intravenously at 75% of the enteral dose.
- The goal of initial L-T4 therapy is to normalize serum FT4 and TSH concentrations as quickly as possible which is attained by early initiation of LT4 tablets as early as 2 weeks.
- Rapid normalization of serum TH levels leads to improved neurocognitive outcomes
- Normalization of serum TSH may lag after the initiation of treatment, particularly in severely affected infants.
- TSH values above 5 mIU/L generally are abnormal if observed after 3 months of age.
- The typical L-T4 starting dose (10–15 mcg/kg per day) frequently results in elevated FT4 levels, so dose reduction is often needed2 weeks after starting L-T4 treatment.
- Some infants with CH have persistent serum TSH elevation despite FT4 levels at or above the upper limit of the reference range. This central resistance to TH may be caused by alteration of pituitary-thyroid feedback caused by intrauterine hypothyroidism.
Monitoring:
Measuring serum TSH and free T4 every 1 to 2 months in the first 6 months of life, every 2 to 3 months in the second 6 months of life, and then every 3 to 4 months between 1 and 3 years of age for children with CH.
Why long term follow up is required?
- Higher risk for neurocognitive and socioemotional dysfunction compared with their unaffected peers, despite adequate treatment of CH.
- Hearing deficits are reported in approximately 10%.
- Negative consequences without treatment.
- Significant proportion of children are lost to follow up and do not continue LT4.
What proportion are permanent CH?
Patients with a eutopic gland are trialed off L-T4 therapy, approximately 40% prove to have permanent CH (low FT4 and high TSH), 25% have persistent hyperthyrotropinemia (normal FT4 and high TSH), and 35% have had transient CH (prior abnormality but now normal FT4 and normal TSH)
When is CH considered permanent?
Thyroid dysgenesis or if the serum TSH increases above 10 mIU/L after the first year of life (on treatment)
Can TSH level at diagnosis predict changes of permanence?
Not necessarily predictive of permanent versus transient disease, and permanent hypothyroidism cannot be assumed solely on the basis of a very elevated TSH level at diagnosis.
What can predict transient hypothyroidism?
- Low L-T4 requirement (particularly below 2 mcg/kg per day) to maintain euthyroidism after 1 year of age.
- Lack of need for increasing L-T4 doses over time, and
- Absence of abnormal TSH levels during treatment
CH due to maternal Graves resolves once TRBAb are cleared from the serum of affected infants, usually by 3 to 6 months of age.

Comments
Post a Comment